Total Hydrocarbon (THC) Analysis in Medical Devices – Technical Principles, Device Construction and Validation Criteria
Total Hydrocarbon (THC) Analysis in Medical Devices – Technical Principles, Device Construction and Validation Criteria
Contents
1. Purpose of THC Analysis in Medical Devices
Total Hydrocarbon (THC) analysis is essential to identify volatile and semi-volatile organic compounds (VOCs) that may leach from medical devices into the patient environment. These compounds may originate from:
-
Lubricants, silicone oils, mold release agents
-
Residual solvents (IPA, hexane, toluene, xylene)
-
Ethylene oxide (EO) sterilization by-products
-
Packaging material emissions
-
PVC, PU, PE, PP, silicone, TPU polymer degradation products
THC analysis is directly related to ISO 10993-18 (Chemical Characterization) and ISO 10993-17 (Toxicological Risk Assessment).
2. Applicable Standards and Guidelines
| Standard / Regulation | Scope |
|---|---|
| ISO 10993-18 | Chemical characterization of medical device materials |
| ISO 10993-12 | Sample extraction (surface area/volume ratio, solvent selection) |
| ISO 10993-5 | Cytotoxicity testing of extracts |
| USP <467> | Residual solvent limits |
| TS EN 12619 / EPA 25A | THC/VOC measurement using Flame Ionization Detector (FID) |
| ISO 13485 / FDA 21 CFR 820 | Process control and documentation |
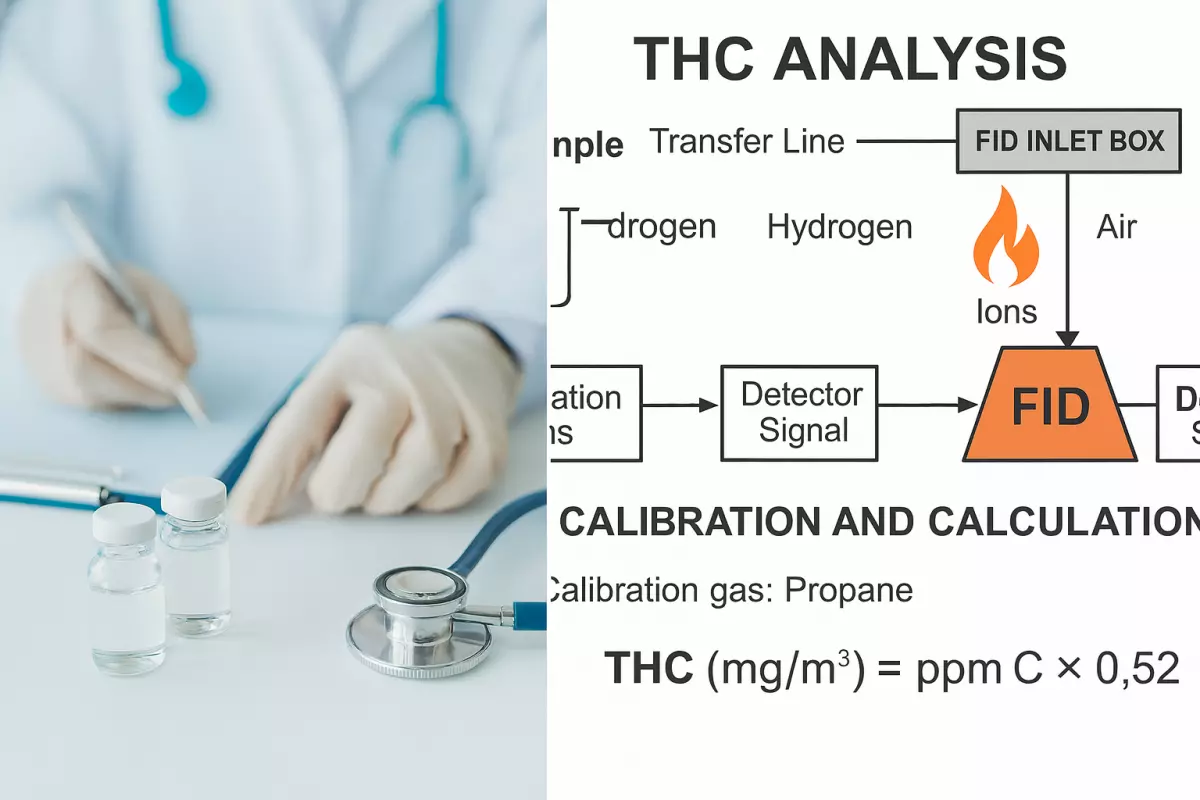
3. Analytical Technique – FID-Based THC Measurement
Flame Ionization Detector (FID) Principle
FID detects organic compounds containing C–H bonds by combusting them in a hydrogen-air flame, producing ions. The ion current is proportional to the hydrocarbon concentration.
| Parameter | Description |
|---|---|
| Flame Gas | Hydrogen + Synthetic Air |
| Detection Limit (LOD) | ~0.05 ppmC |
| Quantitation Limit (LOQ) | ~0.1 ppmC |
| Output Units | ppmC (carbon equivalent), mg/m³ |
4. Sample Preparation – According to ISO 10993-12
| Parameter | Specification |
|---|---|
| Extraction Ratio | 6 cm²/mL (surface area-based extraction) |
| Extraction Media | 0.9% NaCl, Ethanol 70%, PBS, IPA |
| Extraction Temperature | 37°C or accelerated at 50°C |
| Duration | 24–72 hours |
| Filtration | 0.22 μm PTFE filter (low VOC adsorption) |
5. Gas Chromatography – FID Method
GC-FID Operating Conditions
| Setting | Value |
|---|---|
| Column | DB-624 (30 m x 0.32 mm x 1.8 μm) |
| Carrier Gas | Helium (1.5 mL/min) |
| Injection Volume | 1 μL (Split 1:20) |
| Injector Temp. | 200°C |
| Oven Program | 40°C → 200°C at 10°C/min |
| Detector (FID) Temp. | 250°C |
| Hydrogen Flow | 40 mL/min |
| Air Flow | 450 mL/min |
6. Calibration Procedure
Calibration Standards
| Gas Standard | Concentration | Certification |
|---|---|---|
| Propane (C₃H₈) | 1% ±1% | ISO 6142 certified |
| Methane (CH₄) | 1% | Alternative carbon reference |
| Zero Air | Hydrocarbon-free | Baseline correction |
THC Calculation (ppmC to mg/m³)
Example:
Measured Value: 20 ppmC (Propane equivalent)
7. Acceptance Limits for Medical Devices
| Parameter | Limit | Reference |
|---|---|---|
| Total Hydrocarbon Residue | < 10 mg/device | ISO 10993-12 & FDA |
| Residual Solvents (IPA, Hexane) | < 500 µg/device | USP <467> |
| THC in Sterilization Chamber | < 50 ppmC | TS EN 12619 |
| EO Sterilization VOC Residue | < 250 µg/device | ISO 11135 |
8. Method Validation Criteria (FDA & ISO Requirements)
✔ System Suitability Test (SST) performed before analysis
✔ Blank measurement (zero air) < 0.1 ppmC
✔ Linearity: R² ≥ 0.995
✔ Repeatability: RSD < 5%
✔ Recovery: 80–120% (spiked QC samples)
✔ Positive Control: Heptane or Toluene standard solution
9. Reporting Requirements
A compliant test report must include:
-
Sample description (material, batch number, sterilization type)
-
Extraction conditions (time, temperature, solvent, SA/V ratio)
-
GC-FID chromatograms and calibration curves
-
Raw THC values (ppmC) and converted values (mg/m³, µg/device)
-
Comparison with ISO/FDA limit values
-
Risk assessment according to ISO 10993-17
-
Conclusion: Complies / Does Not Comply

