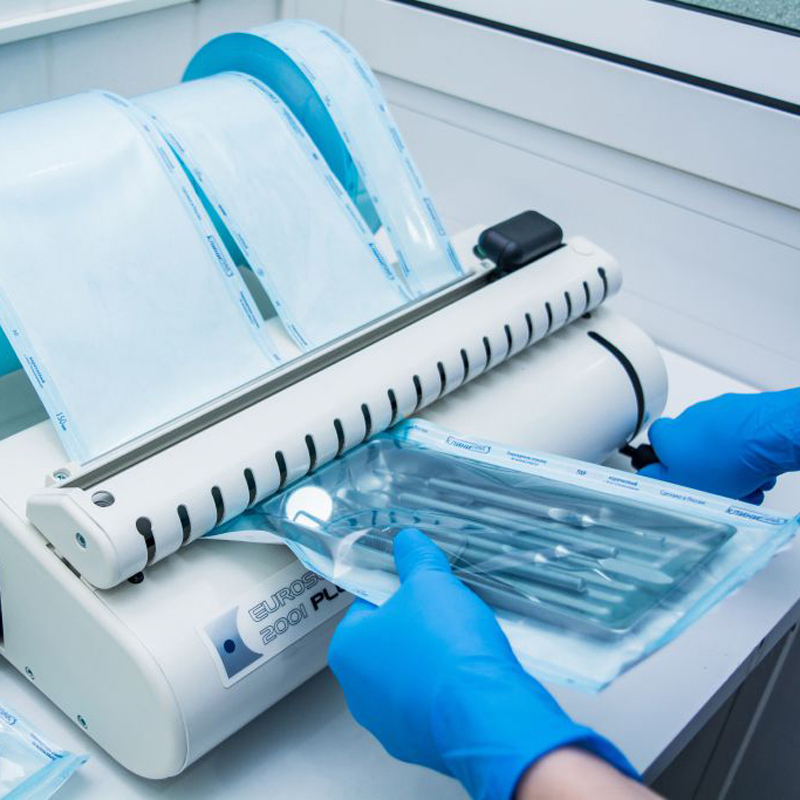
Ethylene Oxide Sterilization Validation
Ethylene oxide sterilization validation tests the effectiveness of the process of sterilizing medical products and inactivating microorganisms.
Test Procedures:
Microbial load tests.
Sterilization cycle control.
Determination of residual ethylene oxide levels.
Standards:
ISO 11135: Requirements for ethylene oxide sterilization.
EN 1422: Design and validation of ethylene oxide sterilizers.
Steam Sterilization Validation
Steam sterilization validation evaluates the process of sterilizing products using pressurized steam. This method is widely used for products such as medical devices and textiles.
Tests:
Biological indicator tests.
Measurement of the parameters constituting the cycle (temperature, pressure, time).
Standards:
ISO 17665-1: Validation of sterilization by moist heat.
EN 285: Performance criteria for steam sterilizers.
Residual Gamma Validation, Dose Control and Dose Determination
In gamma sterilization, dosimetric analysis determines the sterilization effectiveness of the product. Dose control and dose determination are applied to ensure that the applied radiation level inactivates microorganisms without affecting the product structure.
Test Procedures:
Sterilization cycle optimization by radiation dosimetry.
Testing of dose equivalence.
Standards:
ISO 11137-1: Requirements for gamma sterilization dose determination.
ASTM E2303: Gamma radiation dosimetric analysis methods.
Packaging Validation
Packaging validation ensures that medical products remain sterile and resistant to environmental conditions during the packaging process.
Tests:
Durability analysis of packaging material.
Testing of sterile barrier system.
Control of air and moisture leakage.
Standards:
ISO 11607: Packaging requirements and validation.
EN 868: Requirements for sterile barrier systems for medical devices.
Stability Validation
Stability validation tests the shelf life and environmental resistance of medical products.
Tests:
Temperature and humidity resistance tests.
Quality control under storage conditions.
Long-term stability monitoring analyses.
Standards:
ICH Q1A: Stability test requirements.
ISO 2233: Environmental testing of packaged products.
Transport Validation
Transport validation evaluates whether factors such as vibration, impact and temperature changes encountered by products during transport affect product performance.
Tests:
Vibration and impact resistance tests.
Monitoring temperature-humidity fluctuations.
Standards:
ASTM D4169: Transportation durability tests.
ISO 2248: Drop test procedures.
Sterilization Container Validation
Sterilization container validation tests whether containers used in the sterilization process remain sterile without leaking.
Tests:
Air leakage test.
Microbiological barrier control.
Assessment of suitability for sterilization environments.
Standards:
ISO 11607: Container system validation requirements.
EN 868: Sterilization packaging material requirements.
Process Validation
Process validation ensures that manufacturing processes are repeatable and consistent.
Tests:
Determination of critical control points.
Performance assessment of manufacturing equipment.
Document-based quality classification.
Standards:
ISO 13485: Quality management system requirements for medical devices.
Cleaning and Disinfection Effectiveness Validation
This validation tests the microbiological effectiveness of cleaning processes.
Tests:
Surface hygiene control.
Effectiveness tests of cleaning fluids.
Microbial load reduction analyses.
Standards:
ISO 14644-5: Cleaning and hygiene control procedures.
Pure Water Validation
Controls the quality of pure water used in the production of medical products.
Tests:
Microbiological analyses.
TOC (Total Organic Carbon) and conductivity tests.
Standards:
ISO 3696: Laboratory water specifications.
Ultrasonic Cleaning Validation
Controls the hygienic status of equipment cleaned with ultrasonic devices.
Tests:
Cleaning effectiveness tests.
Microscopic particle analyses.
Standards:
ISO 15883: Validation of cleaning and disinfection devices.
Reusable Surgical Instrument Validation
This validation tests the effectiveness of the sterilization and disinfection processes of reusable surgical instruments.
Tests:
Protein and biological residue analyses.
Sterilization procedures appropriate to the instrument structure.
Standards:
ISO 17664: Instructions for reprocessing surgical instruments.
As TTS Laboratory Services, we offer solutions in accordance with international standards with our validation services in medical products.